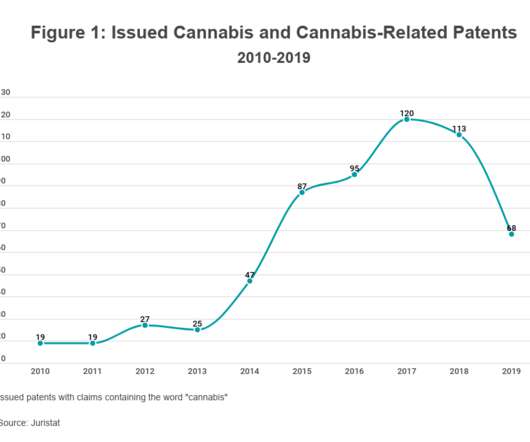
IP and Cannabis: The Current Landscape
Fish & Richardson Trademark & Copyright Thoughts
DECEMBER 2, 2020
As of the date of publication, 36 states and the District of Columbia have legalized cannabis for medical use, while 15 states and the District of Columbia have legalized it for adult recreational use. FDA Regulation. The FDA approved its first drug containing CBD as an active ingredient, Epidiolex, in 2018. THC from Schedule I.















Let's personalize your content