Key Trends in PTAB’s 2021 Orange Book and Biologic Patent Study
LexBlog IP
OCTOBER 27, 2021
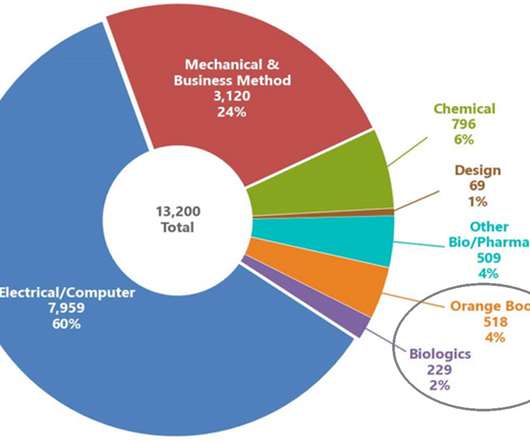
The first study covering these topics was released on March 13, 2018, and the second on July 18, 2019. This is the third report providing data on post-grant petitions filed against Orange Book and biologic patents released by the PTAB, and it covers AIA petitions filed between September 16, 2012 and June 30, 2021. Trends Over Time.










Let's personalize your content