On November 15th, the Federal Circuit handed down its opinion affirming all aspects of the District Court's decision in Pharmacyclics LLC v. Alvogen, Inc. The case illustrates once more the importance of the substantial evidence standard in support of factual aspects of a district court's decision, even regarding ultimate questions of law, such as enablement and obviousness that are based on factual considerations.
The case arose as ANDA litigation between Pharmacyclics LLC and Janssen Biotech, Inc., whose patents for the compound ibrutinib (an inhibitor of Burton's tyrosine kinase (BTK) and the basis for their Imbruvica Product) was challenged by Alvogen and Natco Pharma Ltd. The drug is used for treatment of immune system cancer, specifically relapsed/refractory mantle cell lymphoma (RR/MCL). At trial, Pharmacyclics asserted five claims:
Claim 10, U.S. Patent No. 8,008,309 ("the '309 patent"):
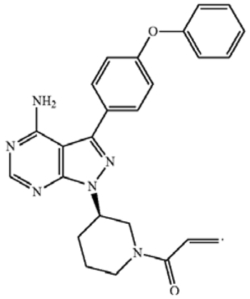
10. The compound of claim 1 [which claims a genus of BTK inhibitor compounds] having the formula 1-((R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one.
Claim 2, U.S. Patent No. 8,754,090 ("the '090 patent"):
1. A method for treating mantle cell lymphoma in an individual who has already received at least one prior therapy for mantle cell lymphoma comprising administering to the individual once per day be- tween about 420 mg to about 840 mg of an oral dose of an inhibitor of Bruton's tyrosine kinase (Btk) having the structure:
2. The method of claim 1, wherein the once per day oral dose is about 560 mg.
Claim 5, U.S. Patent No. 9,725,455 ("the '455 patent") :
1. A crystalline form A of [ibrutinib] that has an X-ray powder diffraction (XRPD) pattern comprising 2-Theta peaks at 5.7±0.1o, 18.9±0.1o, and 21.3±0.1o.
5. The crystalline form of claim 1, wherein the X- ray powder diffraction (XRPD) pattern further comprises 2-Theta peaks at 13.6±0.1o, 16.1±0.1o, and 21.6±0.1o.
Claim 30, U.S. Patent No. 9,655,857 ("the '857 patent"):
30. The high-load solid tablet formulation of claim 1 [which recites a genus tablet formulation for ibrutinib], consisting essentially of:
a) about 70% w/w of ibrutinib,
b) about 14% w/w of lactose monohydrate,
c) about 5% w/w of microcrystalline cellulose,
d) about 2% w/w of polyvinylpyrrolidone,
e) about 7% w/w of croscarmellose sodium,
f) about 1% w/w of sodium lauryl sulfate,
g) about 0.5% w/w of colloidal silicon dioxide, and
h) about 0.5% w/w of magnesium stearate.
Claim 37, the '857 patent:
37. The solid tablet formation of claim 27 [which recites a genus tablet formulation for ibrutinib in an amount of about 70 mg to about 840 mg] consisting essentially of
a) about 69% w/w to about 71% w/w of ibrutinib,
b) about 13% w/w to about 15% w/w of lactose monohydrate,
c) about 2% w/w to about 5% w/w of microcrystalline cellulose,
d) about 1% w/w to about 3% w/w of polyvinylpyrrolidone,
e) about 6% w/w to about 8% w/w of croscarmellose sodium,
f) about 1% w/w to about 4% w/w of sodium lauryl sulfate,
g) about 0.4% w/w to about 0.6% w/w of colloidal silicon dioxide, and
h) about 0.4% w/w to about 0.6% w/w of magnesium stearate.
The parties stipulated to infringement of the asserted '309, '090, and '455 patent claims and the District Court found Alvogen's proposed generic ibrutinib would infringe the asserted claims of the '857 patent. The District Court found against Alvogen on all arguments of invalidity (only some of which were the subject of this appeal, and none of these other arguments raised any additional significant issues). Alvogen and Natco appealed.
The Federal Circuit affirmed, in an opinion by Judge Bryson joined by Judges Hughes and Chen. The opinion addressed each ground of appeal raised against each asserted claim seriatim, noting initially that in an appeal from a bench trial the Court reviews factual determinations for clear error, citing UCB, Inc. v. Watson Lab'ys Inc., 927 F.3d 1272, 1286 (Fed. Cir. 2019), for the principle and Biogen Int'l GmbH v. Mylan Pharms. Inc., 18 F.4th 1333, 1341 (Fed. Cir. 2021), for the standard (i.e., that the Court finds clear error only when it has a "definite and firm conviction that a mistake has been made").
Regarding claim 2 of the '090 patent, the opinion addresses the District Court's finding that this claim was supported by an adequate written description, with an enabling specification, and was not obvious. The basis for the Court's affirmance of an adequate written description was that the '090 specification disclosed two related clinical trial protocols for using a BTK inhibitor to treat R/R MCL. The first of these taught using a variety of such inhibitors at dosages based on patient weight, and the other used a broader range of such inhibitors as a specific dosage ("about 560 mg/day). In addition, the Summary of the Invention section of the patent expressly disclosed ibrutinib for treating R/R MCL. Applying its "blazemarks" analysis (see Novozymes A/S v. DuPont Nutrition Biosciences APS, 723 F.3d 1336, 1346 (Fed. Cir. 2013) (quoting In re Ruschig, 379 F.2d 990, 994–95 (C.C.P.A. 1967)), the Court held it was not clearly erroneous for the District Court to find an adequate written description where ibrutinib was "'the only BTK inhibitor identified by name in the Summary of the Invention and is the only BTK [inhibitor] identified for the treatment of R/R MCL' in the '090 patent." The Court expressly distinguished the circumstances here from its decision in Biogen (a failure to satisfy the written description requirement) because there the only disclosure of the claimed dosage was as one end of a broader disclosed range (in the context of "a long series of ranges") whereas here the claimed dosage was "expressly recited by itself" both in the claim and the specification as filed. (Undiscussed were the decidedly different circumstances arising during prosecution of the patent-in-suit in Biogen supporting the Court's opinion.) Regarding Alvogen's similar arguments on enablement, that the specification disclosed exactly 560 mg/d and claimed "about" 560 mg/d, the Federal Circuit found no clear error in the District Court's determination that there was sufficient disclosure in the specification for the skilled artisan to follow the disclosed protocol and practice the claimed method.
As for obviousness, the Federal Circuit rejected Alvogen's argument that the District Court incorrectly determined that the skilled worker would not have been motivated to treat R/R MCL with ibrutinib from prior art references teaching treatment of MCL with the drug, in light of the District Court's finding of fact that disclosure of treating MCL would not be interpreted by the skilled worker as evidence of effective treatment of RR/ MCL. The panel also refused to find error in the District Court's finding that disclosure of two R/R MCL patients having experienced a "partial response" to ibrutinib in a press release, in view of the small sample size and the propensity for oncology drugs to have a low frequency of receiving FDA approval ("less than five percent of oncology drugs that enter a Phase I trial ultimately receive FDA approval"). Another asserted and rejected obviousness argument was that the skilled worker was capable of finding the recited dose (560 mg/d) as a therapeutically effective amount by routine experimentation, based on evidence that "typical" dose escalation studies would have involved dosages greater than 560 mg/d and would require "a study using pharmacodynamic endpoints" that was not disclosed in Alvogen's combination of references. With regard to the motivation to combine, the panel recognized that Alvogen's expert testified to safety concerns in 2006 rather than 2010 (when the application was filed) and Pharmacyclics asserted contrary expert testimony that the District Court found persuasive. The panel also affirmed the District Court's decision regard a "presumption of obviousness" from the cited prior art teachings, on two grounds. The first was that such a presumption is proper "when the only difference from the prior art is a difference in the range or value of a particular variable," citing In re Kumar, 418 F.3d 1361, 1366 (Fed. Cir. 2005), which was not the case here, and second that Pharmacyclics "would have rebutted any [such] presumption." Finally, the Court did not reach the question of secondary considerations because the District Court's non-obviousness determination made this argument unnecessary.
Turning to claim 5 of the '455 patent, the Federal Circuit affirmed the District Court's determination that the claim was neither inherently anticipated nor obvious over the cited art. Alvogen's inherent anticipation argument was grounded in an assertion that the Form A polymorph of ibrutinib (recited in claim 5) was the only polymorph used in clinical trials disclosed in the art, wherein Alvogen relied upon Abbott Laboratories v. Geneva Pharmaceuticals, Inc., 182 F.3d 1315 (Fed. Cir. 1999) (an on-sale bar case). The Federal Circuit distinguished the circumstances here from those in the Abbott case, and cited Schering Corp. v. Geneva Pharms., 339 F.3d 1373, 1377 (Fed. Cir. 2003), as the more apt precedent. According to the opinion, the circumstances before the District Court in this case were more analogous to those in Endo Pharms. Sols., Inc. v. Custopharm Inc., 894 F.3d 1374, 1382 (Fed. Cir. 2018), upon which the District Court relied, there being no evidence in this case that the only therapeutically effective polymorph of ibrutinib was Form A, and that the District Court's factual determinations in this regard were not clearly erroneous. As for Alvogen's obviousness arguments, the Federal Circuit affirmed the District Court based on, inter alia, there being no clear error in that court's reliance on Pharmacyclics' expert over one of Alvogen's experts (another one agreeing with Pharmacyclics), based on the District Court's appreciation that production of polymorphs and their physical properties was unpredictable. The panel held as not clearly erroneous the District Court's finding that "given the lack of teaching in the art regarding crystalline forms of ibrutinib and the expert testimony that polymorph screening can produce unpredictable results, a skilled artisan would not have reasonably expected success in producing Form A of ibrutinib," citing Grunenthal GmbH v. Alkem Lab'ys Ltd., 919 F.3d 1333, 1344 (Fed. Cir. 2019).
Next, the Federal Circuit held as not clearly erroneous the District Court's finding that claims 30 and 37 of the '875 patent were adequately supported by the written description. Alvogen's argument was that the specification of the '875 patent disclosed one species in a range of species recited in these claims. Alvogen's "problem," according to the Federal Circuit, was that "the precise ranges recited in the claims are found in formulations disclosed in the specification," and on this basis the Federal Circuit affirmed the District Court's determination that these claims were adequately described.
Finally, the Federal Circuit affirmed the District Court's decision that claim 10 of the '309 patent was not anticipated by the cited art, Alvogen arguing that a skilled worker could not have synthesized a needed intermediate without undue experimentation and accordingly the '309 patent was not entitled to its earliest priority date. Alvogen's assertion of error in this regard was that the District Court should not have relied upon Pharmacyclics' testimony that his undergraduate students could have produced the intermediate without undue experimentation based on the disclosure in the priority documents. The District Court also relied upon the intermediate having been known in a prior art reference upon which the skilled artisan could have relied, and Alvogen argued that the District Court did not apply the proper legal standard for incorporating this document by reference. The panel found this argument not dispositive because "a skilled artisan could have synthesized Intermediate 2 and thus ibrutinib" without reference to the reference. Moreover, the opinion states that "formal incorporation by reference is not necessary if the material being incorporated is background art," citing Falko-Gunter Falkner v. Inglis, 448 F.3d 1357, 1365 (Fed. Cir. 2006). On these grounds, the Federal Circuit held that the District Court committed no clear error in rejecting Alvogen's argument.
To the extent there is any question about the importance of the standard of review in the Federal Circuit's opinion, mere casual perusal thereof finds 15 instances of some version of "clear error" and "clearly erroneous" recited by the Court. A cautionary tale indeed.
Pharmacyclics LLC v. Alvogen, Inc. (Fed. Cir. 2022)
Panel: Circuit Judges Chen, Bryson, and Hughes
Opinion by Circuit Judge Bryson