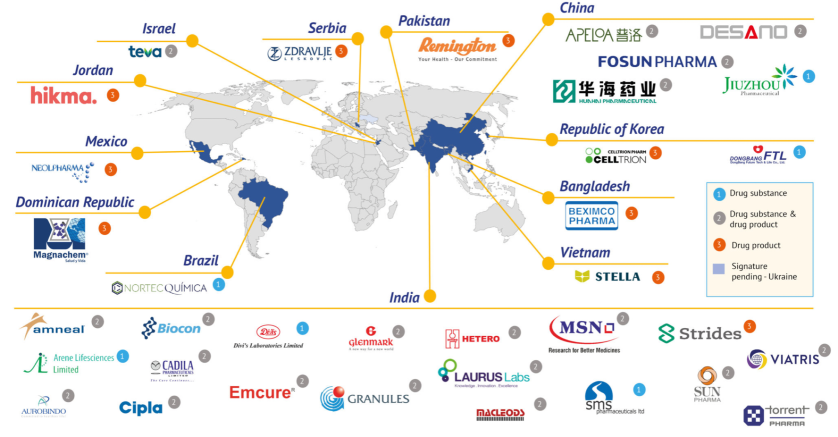
The Medicines Patent Pool (MPP) announced that it has signed agreements with 35 companies to manufacture the generic version of Pfizer’s oral COVID-19 treatment nirmatrelvir, which in combination with a low dose of ritonavir can be supplied in 95 low- and middle-income countries.
The sublicenses are the result of the voluntary licensing agreement signed by MPP and Pfizer in November 2021 that will help enable the supply of the medicines to countries comprising approximately 53% of the world’s population.
- The agreements build on Pfizer’s comprehensive strategy to work toward equitable access of COVID-19 vaccines and treatments for all people, particularly those living in the poorest parts of the world
- Interim data from the Phase 2/3 EPIC-HR study demonstrated an 89% reduction in risk of COVID-19-related hospitalization or death compared to placebo in non-hospitalized high-risk adults with COVID-19 within three days of symptom onset with similar results seen within five days of symptom onset
- Chinese companies have signed five sublicenses, the most of any country, and a license will be granted to a Ukrainian manufacturer which can’t currently sign the agreement due to the Russian invasion of the country.
Many of the world’s poorest nations have the lowest Covid vaccine adoption. Anti-virals like Pfizer’s Paxlovid, taken at the early onset of Covid-19 symptoms, have been proven to be highly effective at preventing hospitalization.
“[Poorer nations] have been at the back of the queue for vaccines, so having a treatment like this in the armory will be absolutely critical to prevent deaths.”
“This will make an enormous difference for countries,” said Charles Gore, executive director of the Medicines Patent Pool. He stated that the availability of the Pfizer drug in some of the world’s poorest countries is especially critical. “They have been at the back of the queue for vaccines, so having a treatment like this in the armory will be absolutely critical to prevent deaths.”
Slow U.S. Rollout
Rollout of Paxlovid in the U.S. has been slow, with many who contract Covid unaware they qualify for receiving it and when in the course of the virus it should be taken. With the medication now more abundant, pharmacists, public health experts and state health officials say that encouraging the right people to take Plaxlovid, and making it easier for them to access, could help blunt the effects of another Covid wave.
“State health officials believe that many Americans who would be good candidates for Paxlovid do not seek it out because they are unaware they qualify for it,” reports The New York Times. They are hesitant about taking a new medication, or confused by the fact that some providers interpret the eligibility guidelines more narrowly than others.
Gore of MPP estimates in U.S. News and World Report that some of the generic companies might be ready to submit their drugs for regulatory approval later this year, with some supplies available in 2023. Many of the anti-viral drugs are currently available in the U.S. and Europe.
Image source: MPP; abcnews.go