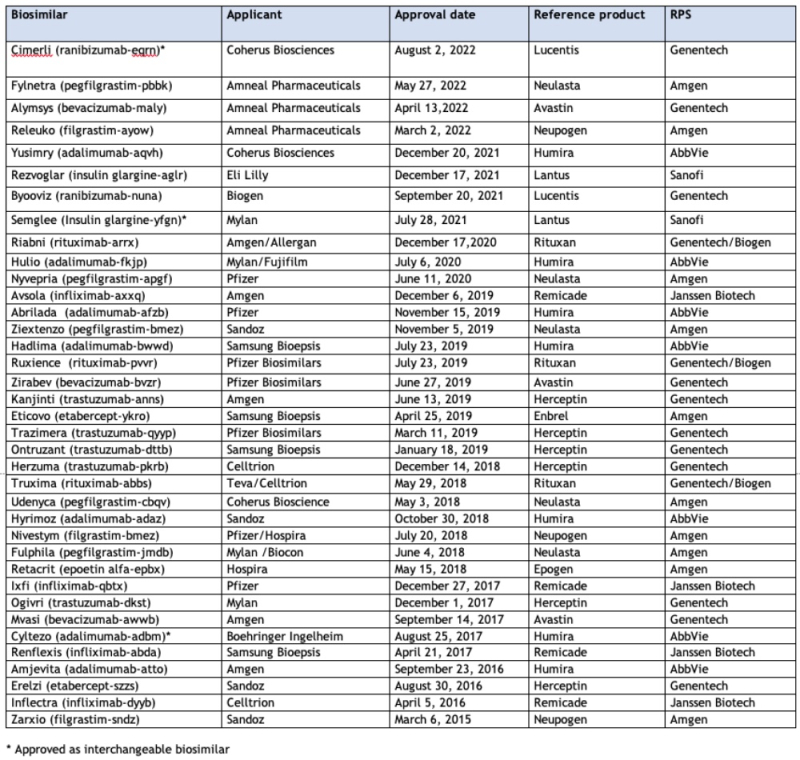
On August 2nd, Coherus Biosciences announced FDA approval for its Cimerli™ (ranibizumab-eqrn) product, as an interchangeable biosimilar to Genentech/Roche's Lucentis® (ranibizumab). This biologic drug is a vascular endothelial growth factor (VEGF) inhibitor having five approved indications for Lucentis: the retinal diseases age-related macular degeneration, retinal vein occlusion, diabetic macular edema, diabetic retinopathy, myopic choroidal neovascularization. Cimerli™ has been approved for all of the Lucentis®-approved inductions. FDA approval of Cimerli™ as an interchangeable biosimilar was attributed by Coherus Biosciences to the outcome of a clinical trial, the COLUMBUS-AMD study.
The FDA has approved another biosimilar, Byooviz (ranibizumab-nuna) from Biogen/Samsung Bioepsis in September 2021 but for only three of the five indications. As an interchangeable biosimilar, Cimerli™ is entitled to a one-year term of interchangeable exclusivity, which can be expected to have significant economic benefits in the annual $7 billion anti-VEGF ophthalmology market. Coherus Biosciences is expected to launch Cimerli™ in October 2022 in both 0.3 mg and 0.5 mg dosages.
This approval brings to 37 the number of approved biosimilar products and is the third interchangeable biosimilar to be approved (the others being Semglee (insulin glargine-yfgn) and Cyltezo (adalimumab-adbm)).
[View source.]