The Federal Circuit recently affirmed a district court judgment of invalidity for obviousness and for noninfringement for a series of patents challenged in ANDA litigation, in Genentech Inc. v. Sandoz Inc. In doing so, a majority of the panel illustrated perhaps unintentionally how initial impressions regarding the issues before the Court can influence the final outcome in circumstances where the Federal Circuit is compelled to give deference to the lower court.
These issues arose in ANDA litigation brought by Genentech and InterMune against ANDA filer Sandoz and its supplier LEK Pharmaceuticals. The drug at issue was pirfenidone used to treat idiopathic pulmonary fibrosis (IPF), an incurable, chronic, irreversible disease whose patients have a 2- to 5-year average survival length. There is one other drug, nintedanib that has been approved for treating IPF and the two drugs split the marker evenly (approximately). Pirfenidone had a long development time (more than 30 years) before obtaining orphan drug approval in 2004 and was approved for IPF treatment in 2014 as Esbriet®, the drug sold by Genentech and the proximal subject of this lawsuit.
Unlike most ANDA suits, the patents at issue here were not directed at the drug, formulations thereof, or methods of treatment, but rather towards administration methods for ameliorating or avoiding certain drug side effects. There were two categories of patents at issue in the ANDA litigation and this appeal. The first, termed the "LFT patents," were directed at administration methods for patients having liver abnormalities as detected by biomarker alterations. These patents were U.S. Patent Nos. 7,566,729 (the "'729 patent"), 7,635,707 (the "'707 patent"), 8,592,462 (the "'462 patent"), and 8,609,701 (the "'701 patent") and the claims at issue are set forth in this table:
The asserted LFT patent claims:
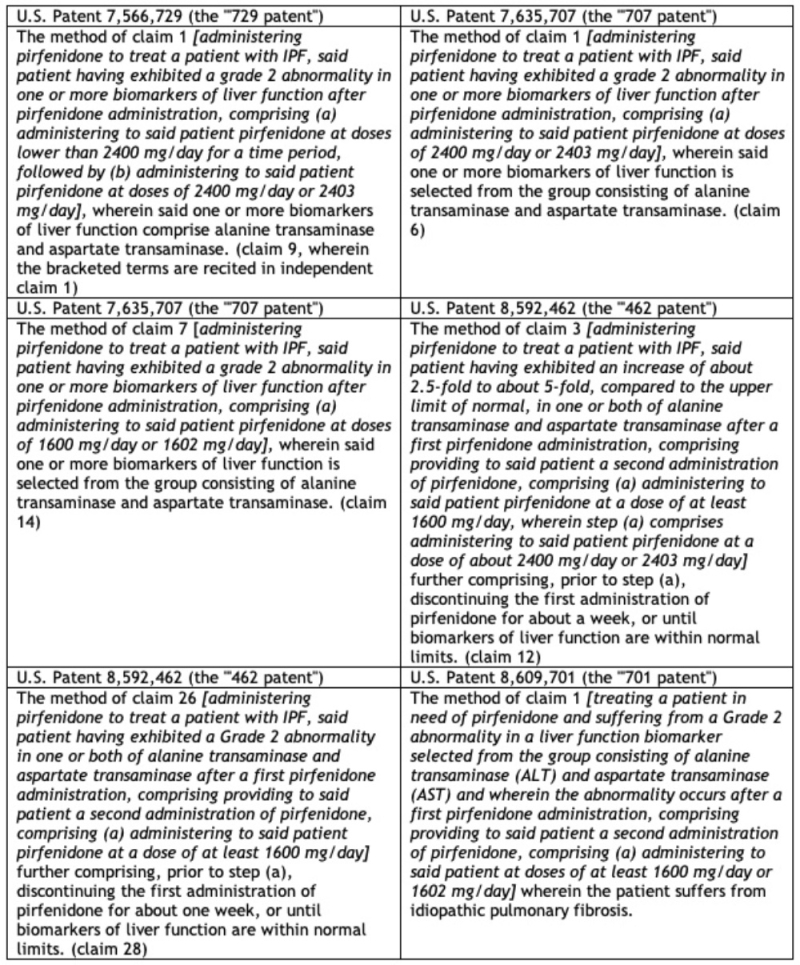
The opinion summarizes the claimed subject matter into five categories: administration methods that (1) temporarily reduce the administered dose followed by return to the "full" therapeutic dose; (2) maintain the full administered dose; (3) reduce the dose (and maintain the reduced dose); (4) discontinue administration for a week and then return to the full therapeutic dose; and (5) discontinue administration for a week and then administer a reduced dose.
Infringement was assessed as to these claims based on Sandoz's proposed label, which recited how the drug should be administered to patients showing sufficiently severe liver abnormalities:
Dosage modifications or interruptions may also be necessary when liver enzyme and bilirubin elevations are exhibited. For liver enzyme elevations, modify the dosage as follows:
If a patient exhibits >3 but ≤5 x the upper limit of normal (ULN) ALT and/or AST without symptoms or hyperbilirubinemia after starting pirfenidone tablets therapy:
• Discontinue confounding medications, exclude other causes, and monitor the patient closely.
• Repeat liver chemistry tests as clinically indicated.
• The full daily dosage may be maintained, if clinically appropriate, or reduced or interrupted (e.g., until liver chemistry tests are within normal limits) with subsequent re-titration to the full dosage as tolerated.
wherein the italicized language was particularly relevant to Genentech's allegations sounding in inducement of infringement under 35 U.S.C. § 271(b).
Sandoz asserted invalidity for obviousness over three references: a scientific journal article by Azuma related to a clinical trial of pirfenidone; a label for Shionogi's pirfenidone product approved in Japan, Pirespa®; and "known, standard medical practices." The Federal Circuit's opinion characterized the teachings of the Azuma reference to specify a "stepwise" reduction in pirfenidone administration up to and including discontinuing the drug in response to severity of liver-associated side effects, and that the Pirespa® label specified discontinuing the drug. The District Court had found that four of the five liver-associated side-effect induced alterations in pirfenidone administration were disclosed in the Pirespa® label, and furthermore found that Genentech's asserted claims in the LFT patents were invalid for obviousness in light of the references relied upon by Sandoz.
Sandoz also argued that there was no specific intent to induce infringement and thus its label did not induce infringement.
The other category of patents asserted by Genentech were directed to adverse interactions with another drug, fluvoxamine (termed "the DDI patents"). Fluvoxamine inhibits an enzyme, CYP1A2, that is involved in liver detoxification and metabolism of drugs including pirfenidone, where administration of pirfenidone to a patient taking fluvoxamine would result in what the opinion calls "supertherapeutic" drug levels in the patient's body. The claims at issue for these patents were as follows:

The opinion summarizes the instructions in Sandoz's label (which forms the basis for Genentech's direct (literal) infringement allegations) as directing that fluvoxamine administration be discontinued prior to administering pirfenidone or reducing the amount of pirfenidone administered to a specified dose (267mg/3 time per day). The District Court found this label to be insufficient to support Genentech's direct infringement allegations by a preponderance of the evidence because, despite Genentech showing that the label "encourages, recommends, or promotes an infringing use" Genentech failed to show that "such an infringement will in fact occur" (which seems at least odd in view of the function of the label as enshrining FDA determinations regarding how an approved drug can be safely and effectively used). The basis for the District Court's decision was that Genentech failed to show that any patient would be prescribed both drugs to be administered together or at the same time, a prohibition that if it deemed necessary, the FDA would be capable of requiring, particularly insofar as the label expressly stated that:
The concomitant administration of pirfenidone and fluvoxamine or other strong CYP1A2 inhibitors (e.g., enoxacin) is not recommended because it significantly increases exposure to pirfenidone. . . . Use of fluvoxamine or other strong CYP1A2 inhibitors should be discontinued prior to administration of pirfenidone and avoided during pirfenidone treatment. In the event that fluvoxamine or other strong CYP1A2 inhibitors are the only drug of choice, dosage reductions are recommended. Monitor for adverse reactions and consider discontinuation of pirfenidone as needed [emphasis added].
The Federal Circuit affirmed, in an opinion by Judge Lourie who was joined by Judge Prost; Judge Newman dissented without an opinion (perhaps getting to the point where the Judge is not willing to waste her judicial breath in cases where her opinion has proven to be unavailing). With regard to the District Court's finding that the asserted claims of the LFT patents were obvious, the majority displayed the mindset brought to the question by stating that "it is worth noting our initial perception that, as the district court noted, varying doses in response to the occurrence of side effects would seem to be a well-established, hence obvious, practice. Thus, claiming it as an invention would appear to be at best a long shot" before stating that, like the District Court, the Federal Circuit would give the matter "careful scrutiny." Perhaps not surprisingly in view of the majority's preconceptions, the Court agreed with Sandoz that these claims were obvious, despite Genentech's objections regarding what Azuma and the Pirespa® label taught to the skilled artisan (which as questions of fact the District Court's findings were due "clear error" deference by the Federal Circuit). The District Court's findings as to the teachings of the asserted prior art were supported in the majority's view by "record evidence" and expert testimony. Finally, the majority relied upon the Federal Circuit's rubric following KSR v. Teleflex that "weak secondary considerations [as the District Court and the majority considered Genentech's secondary considerations arguments to be] generally do not overcome a strong prima facie case of obviousness," citing W. Union Co. v. MoneyGram Payment Sys., Inc., 626 F.3d 1361, 1371 (Fed. Cir. 2010). These arguments included skepticism in the art and long-felt but unmet need; none of these were sufficiently established by Genentech in the majority's view nor had Genentech established that the District Court clearly erred in arriving at its conclusions in this regard.
Having affirmed the District Court's obviousness determination, the panel did not reach the District Court's non-infringement decision.
With regard to the DDI patents, despite citing precedent that "a patentee does not need to prove an actual past instance of direct infringement by a physician to establish infringement in an ANDA case," Vanda Pharms. Inc. v. W.-Ward Pharms. Int'l Ltd., 887 F.3d 1117, 1129–30 (Fed. Cir. 2018), the majority nevertheless opined that the question of direct infringement requires "'consideration of all the relevant evidence,' including the proposed label's instructions and physician practice," citing Ferring v. Watson Lab'ys, 764 F.3d 1401, 1408 (Fed. Cir. 2014), because "past conduct is relevant to what will happen in the future" (a position the opinion acknowledges Sandoz argued). The majority's affirmance relied upon expert witness testimony that the two drugs (pirfenidone and fluvoxamine) would not be administered together or at the same time based on "decades of treating IPF patients" (which testimony is at least curious in view of the 2014 approval date of Genentech's Esbriet® product) and that there was an alternative product on the market (nintedanib) that the physicians would prescribe instead. The majority characterized as speculation without evidentiary support Genentech's argument that instructions regarding co-administration were important enough that the FDA included them on Sandoz's label, and in any event were not enough to sufficiently support Genentech's direct infringement arguments according to the majority. (And in what appears to be something of a "Hail Mary" argument, the majority rejected Genentech's assertion that fluvoxamine can be used to treat COVID-19 infection in an IPF patient as speculation.)
Based on the totality of the evidence relied upon by the District Court, the Federal Circuit majority affirmed the District Court's finding that Sandoz would not necessarily directly infringe the claims of Genentech's DDI patents.
Genentech, Inc. v. Sandoz Inc. (Fed. Cir. 2022)
Panel: Circui9t Judges Newman, Lourie, and Prost
Opinion by Circuit Judge Lourie; dissent without opinion by Circuit Judge Newman