Pfizer and BioNTech Agree to Vaccine Technology Transfer with South Africa's Biovac Institute
As reported by Reuters* on Wednesday (see "South African firm to help make Pfizer/BioNTech COVID vaccine"), Pfizer and BioNTech, manufacturers and developers of an mRNA-based vaccine against SARS-CoV-2, have agreed to help the South African drug maker Biovac Institute produce about 100 million doses per year of its vaccine, specifically to be targeted to African nations.
As described by the Reuters article, Biovac Institute is "a joint venture between the South African government and private sector partners"; it has an advantage as being the first company in Africa to use mRNA technology to make the vaccine. The scope of the cooperative agreement is limited to "fill and finish" aspects of the vaccine, leaving Pfizer and BioNTech to produce the mRNA components of the vaccine in Europe (for a more detailed description of the vaccine making process, see Lowe, D. "Myths of Vaccine Manufacturing," Science Translational Medicine, February 2021). Production is slated to begin "towards the second half of 2022" according to Biovac CEO Morena Makhoana, with the aspirational 100 million doses per year being achieved in "early" 2023. The value of the agreement is 200 million South African rand ($13.6 million) according to South Africa President Cyril Ramaphosa, who as Reuters reported called the agreement "a breakthrough in efforts to overcome vaccine inequity."
Perhaps also relevant is the World Health Organization's establishment of a consortium (including Biovac) to be a "tech transfer hub" aimed at "giv[ing] poor and middle-income countries the knowledge and licences to produce COVID-19 vaccines.
This is not the first South African company to make such a deal; Aspen has a similar agreement with Johnson & Johnson for its more conventional adenovirus-based COVID-19 vaccine. This is also not the first vaccine-related partnering agreement between Pfizer and Biovac, who in 2015 agreed to collaborate on Pfizer's pneumonia vaccine Prevnar; distribution of the vaccine has been delayed in South Africa due to regulatory approval requirements.
The WTO proposal (see Communication IP/C/W/669, "Waiver from certain provisions of the TRIPS agreement for the prevention, containment and treatment of COVID-19, 2 October 2020) has been vigorously promoted by many countries (most of which lack the production capacity to make the vaccine) and opposed predominantly by European countries, as illustrated by this map:
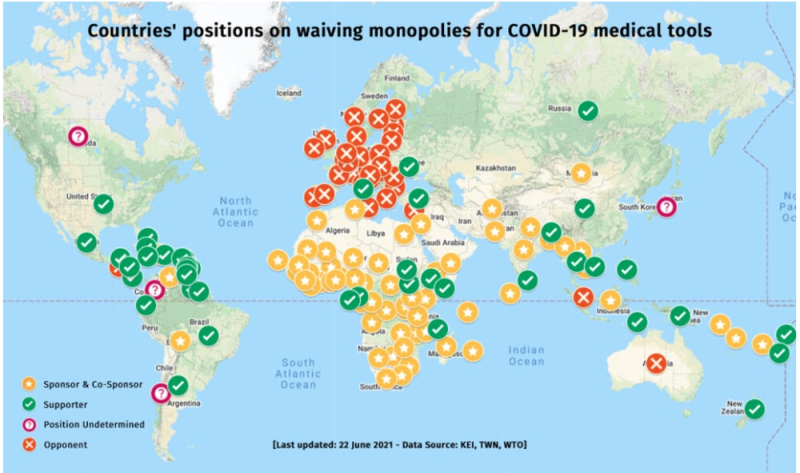 https://www.msf.org/countries-obstructing-covid-19-patent-waiver-must-allow-negotiations
https://www.msf.org/countries-obstructing-covid-19-patent-waiver-must-allow-negotiations
The progress of approval of the waiver is uncertain (see "If the Devil of the WTO IP Waiver Is in the Details, What Are the Details?") and the wisdom even more so (see "Suspending IP Protection: A Bad Idea (That Won't Achieve Its Desired Goals"). In addition to the EU, most companies involved in producing vaccines and other pharmaceuticals have opposed the waiver (see "Pfizer CEO Pens Open Letter on COVID-19 Vaccine IP Waiver") as have IP and industry groups (see "IP Organizations Support Continued Opposition to Waiver Proposal; Industry Coalition Supports Continued Efforts to Oppose Waiver Proposal"; "BIO and PhRMA Urge Biden Administration to Oppose Proposed WTO TRIPS Waiver"; "Industry Coalition Supports Continued Efforts to Oppose Waiver Proposal"; and "IPO Sends Letter on IP Law and Policy to President-Elect and Vice President-Elect") and politicians and policymakers (see "Sen. Tillis Asks Biden Administration to Oppose WTO Waiver Proposal"; "GOP Legislators Write in Opposition to Proposed TRIPS Waiver"; "Sen. Daines Urges Biden Administration to Withdraw Support for COVID-19 IP Waiver"; Andrei Iancu, former U.S. Patent and Trademark Office Director, quoted in StatNews; Reto Hilty, Director at the Max Planck Institute for Innovation and Competition, https://www.mpg.de/16579491/patent-protection-vaccines-covid-10-reto-hilty).
The negative consequences of the inequities between the developed world and everyone else on both global public health and the intellectual property regime are not now just being appreciated (see, e.g., "A Modest Proposal Regarding Drug Pricing in Developing Countries"; "The Law of Unintended Consequences Arises in Applying TRIPS to Patented Drug Protection in Developing Countries"; "Africa (Still) Depending on the Kindness of Strangers in Anti-AIDS Drug Pricing"; and "Worldwide Drug Pricing Regime in Chaos") but like many issues the COVID-19 pandemic has raised the temperature on the debate. Perhaps the latest developments were the goal of the countries promoting the IP waiver at the WTO all along. Also possible is that continued resistance from European countries to the waiver has convinced waiver proponents to use the leverage they have due to the pandemic to move the needle in the direction of technology transfer as far as they can (or at least create a precedent for it for vaccines). Long term this development is likely to be more positive than merely having low- and middle-income countries rely on vaccine donations as the Biden Administration and others have pledged (such donations are put in context by the Kaiser Family Foundation in Michaud et al., "Putting U.S. Global COVID-19 Vaccine Donations in Context," May 25, 2021). But whether this will be enough to reduce pressure on the industrialized world to reduce or eliminate the IP requirements on less economically developed countries created by the GATT/TRIPS/WTO regime remains to be seen.
*Reporting by Michael Erman, Wendell Roelf, and Alexander Winning; editing by Richard Pullin and Mark Potter