During oral argument before the Supreme Court on Monday in Amgen v. Sanofi, all three advocates (Jeff Lamken for Amgen, Paul Clement for Sanofi, and Colleen Sindzak for the United States) had reason to reference and discuss an amicus brief submitted on behalf of Nobel Prize-winning scientist Sir Gregory Winter and colleagues,* on the scientific questions raised in the case with regard to what is sufficient to satisfy the enablement requirement of 35 U.S.C. § 112(a).
The brief first extols the success of antibody technology as treatments for auto-immune inflammatory diseases and cancer that "has revolutionized the pharmaceutical industry." It characterizes Amgen's position as being that "because it was the first to determine the identity of the amino acids that make up the natural site on PCSK9 where LDL receptors bind (the alleged 'sweet spot') it should be entitled to patents covering any and all antibodies that bind there." The scientists take issue with this assertion, as they have characterized it, because "Amgen did not invent the natural binding site, nor did Amgen even use its discovery of that alleged 'sweet spot' to make its own two lead antibodies," and contend that Amgen's invention is merely "a hindsight characterization of that which existed naturally. Amgen attempts to monopolize the natural PCSK9 binding site by reciting the specific amino acid residues of that site." Moreover, the scientists criticize Amgen for not teaching how to make and use the invention they have claimed.
The scientists state that they have filed their amicus brief to "provide[] information and scientific perspectives concerning several issues at the heart of this case"; these are:
"(1) the unpredictability of antibody design and engineering, including methods of generating and testing antibodies for a particular function;
(2) the lack of guidance provided by Amgen's patents, and indeed, the additional hurdles created by Amgen's claims to make and use the claimed class of antibodies; and
(3) the devastating impact of overbroad, purely functional claims like Amgen's on antibody development and innovation for pharmaceutical drugs."
An antibody's structure (its amino acid sequence) determines its structure ("a fundamental tenet of basic antibody science") but the reverse is not true, according to the brief. Thus, knowing an antibody's function (such as binding PCSK9 at the "sweet spot") does not provide any information about its structure (which the scientists assert is "what it is," emphasis in the brief). In addition, Amgen's claims do not encompass "a narrow class of specific antibodies," the scientists assert, but are broadly recited to encompass "any and all antibodies (of unspecified and unknown structure) that bind to a natural antigen at its natural interface with natural receptors." Referencing undue experimentation (a bit obliquely in the argument), the scientists contend that these claims add to the undue burden of identifying PCSK9-binding antibodies by necessitating that scientists must "make, test, and characterize each one of potentially billions of antibodies to determine whether they are covered by Amgen's claims," i.e., bind to these specific residues in the sweet spot. And permitting this situation to stand (rather, resurrecting it by reversing the Federal Circuit) would "stifle innovation and set a dangerous precedent for the scientific and pharmaceutical community at large."
The brief then dissects the scientific bases for its arguments. After providing an "overview" of antibody structure and design, including the graphic:
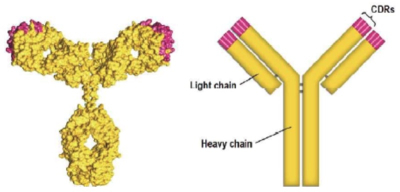
and the amino acid sequence of the heavy chain variable region comprising CDRs from one of Amgen's antibodies:

the scientists assert that as a consequence of the complexity of antibody structure, antibodies having different amino acid sequences can bind to PCSK9, as illustrated by Respondent's "demonstrative exhibit" (as it was called by Justice Gorsuch during oral argument):
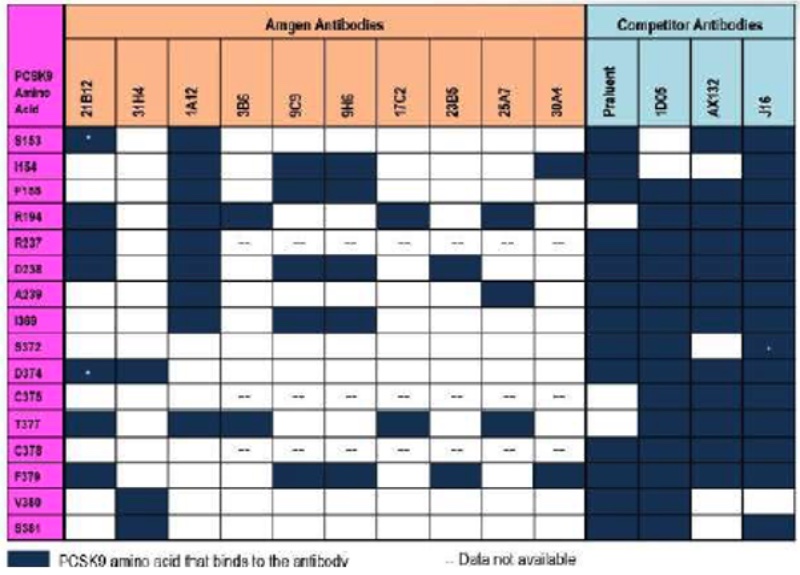
(which illustrates, importantly for Respondents' arguments, that while Amgen's antibodies contact only 2-3 amino acids in the sweet spot, the "competitor antibodies" contact nearly all of them, due purportedly to the different methods by which the competitors produced them). These structural differences have functional consequence, according to the scientists, as illustrated by the topography of where Sanofi/Regeneron's Praluent antibody binds PCSK9:
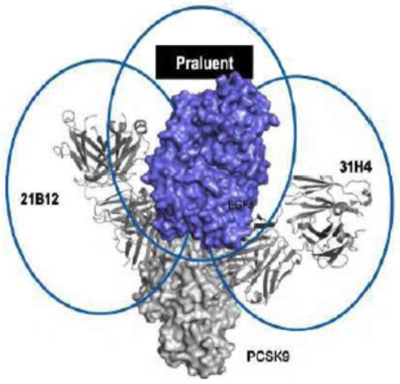
(where "21B12" and "31H4" are Amgen's antibodies disclosed in Amgen's patent that bind on either side of the site to which Praluent binds).
The brief states that an antibody's amino acid sequence is the "recipe" for the antibody (a description used by the U.S. during oral argument) because it determines both what an antibody is and what it does (its function), which is dependent on "precise order of amino acids [that] dictates how they will interact, how the chains will fold and arrange, and, additionally, which amino acids will comprise the CDR loops that ultimately interact with antigens." Consequently, according to the brief, changing even a single amino acid in the sequence "could turn an antibody that binds to an antigen into an antibody that does not bind to that same antigen" as attested at trial by Amgen's expert (a property that can be shared with chemical entities, the scientists asserts in a footnote). As a consequence, the scientists remind that it is unpredictable how an amino acid sequence change will influence function (in an antibody's case, what it binds to and how well). The scientists use this unpredictability to criticize Amgen's reliance on "conservative substitutions" as somehow enabling a scientist to modify the antibodies whose sequences are disclosed in Amgen's patent to produce other antibodies having the same PCSK9 binding capabilities. The scientists attest that conservative substitutions "are by no means a 'shortcut' in antibody engineering for therapeutics" because "the impact of such substitutions remains highly unpredictable," the brief going on to criticize some of Amgen's assertions about such conservative substitutions. "The only sure way to determine whether the function of an antibody tolerates an amino acid substitution is to make the substitution and test the resulting antibody" according to the scientists. Nothing about an antibody is predictable, from having an antibody having the desired function to changing portions of the amino acid sequence of such an antibody, even by conservative substitutions.
The logical conclusion advanced by the scientists is that this feature of antibody structure makes obtaining antibodies other than those expressly disclosed by Amgen undue experimentation. The degree of experimentation is more, not less, for antibodies that must bind to the identified amino acids in the PCSK9 sweet spot according to the brief.
The brief then makes the argument that Amgen's invention is the result of hindsight and that the elements (the existence of the sweet spot) was not invented by Amgen (nor according to the scientists did Amgen discover PCSK9 or how LDL receptors bind to it, the brief identifying by name those scientists who did, none of which "had any connection with Amgen"). And the scientists affirm Respondents' argument that Amgen's claims do not encompass a small genus but rather "cover the entire genus of antibodies that bind to specific amino acid residues on PCSK9 and block PCSK9 from binding" to the LDL receptors (quoting the Federal Circuit), despite not reciting any amino acid sequence limitations. (The brief spends a considerable number of pages addressing and rebutting analogies made by Amgen and other amici.)
At the end of their legal arguments, the scientists assert that Amgen's disclosure does not satisfy the statutory "make and use" requirement because they "do not come close to teaching an antibody scientist how to make and use antibodies that bind to its claimed specific residues and block binding of LDL receptors." With regard to Amgen's purported "roadmap" the scientists state that "[n]owhere in this 'roadmap' is any teaching or guidance that provides a practical shortcut to other scientists to make and use the claimed broad genus of antibodies without undue experimentation."
The brief ends on a policy note, the scientists asserting that letting Amgen's claims stand would "block innovation and access to new medicines." Amgen provided the two expressly recited antibodies (defined by structure) but did not "create or alter any of these natural residues; they existed in nature before Amgen found them." This strikes (for the scientists) the "warning bell" that permitting claims having Amgen's scope would inherently give Amgen patent protection over the naturally occurring structure of PCSK9, contrary to Ass'n for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. 576, 589-90 (2013). Having an enablement standard that would permit disclosures such as Amgen's here to pass muster would "incentivize companies to use their considerable resources to block access to new therapies by essentially calling 'dibs' on anything that binds to a naturally occurring target of interest" according to the brief. These scientists rebut arguments by Amgen's amici that claims like Amgen's promote innovation and state that the opposite is true, that "an arms race to patent natural interfaces or surfaces of targets involved in key interactions in a disease undermines scientific development and gives an unfair advantage to companies with unlimited resources."
The brief ends with this summary statement:
In sum, purely functional claims like Amgen's are enormously harmful to scientists who seek to understand, research, and develop new therapies for known targets, particularly where the technology at issue is complex and unpredictable. No entity should be able to contribute only a few drops and claim ownership of the ocean. The current enablement standard protects innovation and limits exclusivity to that which is truly inventive. It should be upheld.
*This group of scientists were Sir Gregory Winter, who won a Nobel Prize in 2018 for methods of making fully human recombinant antibodies by antibody phage display technology; Timothy Springer, Ph.D., the Latham Family Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School and Boston Children's Hospital, as well as the Principal Investigator in the Program of Cellular and Molecular Medicine, Division of Hematology/Oncology, Department of Medicine at Boston Children's Hospital, whose work has been in integrins; Robert Kamen, Ph.D., who is an advisory partner at Third Rock Ventures; Andrew Griffith, Ph.D., Professor of Biochemistry at École Supérieure de Chimie Industrielles de Paris (ESPCI Paris) in Paris and a founder of Cambridge Antibody Technology; Royston Jefferis, Ph.D., who is Professor Emeritus in the Institute of Immunology and Immunotherapy within the College of Medical and Dental Sciences at the University of Birmingham, UK, whose work is on antibody design; Nick Ray, Ph.D., Chief Scientific Officer of a UK drug discovery company, C4X Discovery, who has worked on oncology, respiratory diseases, inflammation, central nervous system, pain and metabolic disease and is a named inventor on over 75 patents; and David Manuta, Ph.D., current Board Chair of the American Institute of Chemists (AIC), "a national, non-profit organization founded in 1923 for emphasizing and promoting the relevance of the chemical profession and its practitioners to society at large."