Battle of the experts: court deals with surveys, damages, other Lanham Act experts
43(B)log
FEBRUARY 17, 2022
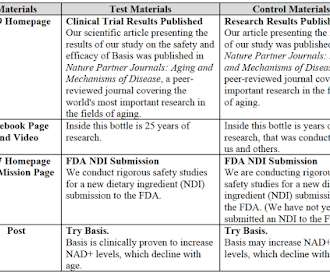
Elysium’s survey/expert was not excluded on Daubert grounds, but the court rejected Elysium’s challenges to ChromaDex’s statements “that do no more than convey the FDA status of ChromaDex’s products,” so the survey was no longer relevant. The expert concluded that 23.3%









Let's personalize your content