Federal Circuit Clarifies Public Use Bar Requirements in Win for Hologic Against Minerva
IP Watchdog
FEBRUARY 15, 2023
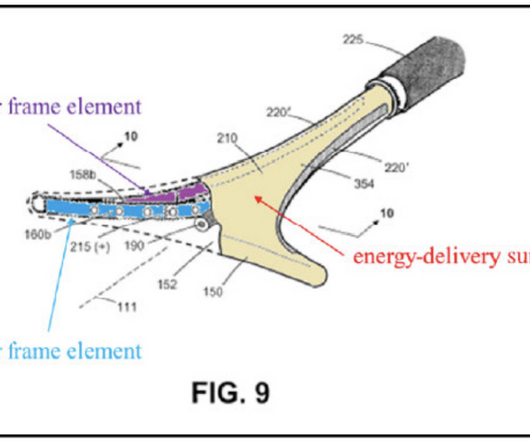
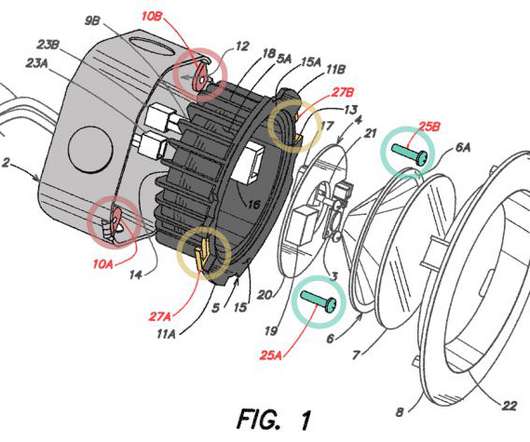
Court of Appeals for the Federal Circuit (CAFC) on Wednesday issued a precedential opinion clarifying the requirements for the disclosure of technology that is ready for patenting at a public event to qualify as being “in public use” for purposes of the pre-America Invents Act (AIA) public use bar under 35 USC 102(b).
















Let's personalize your content