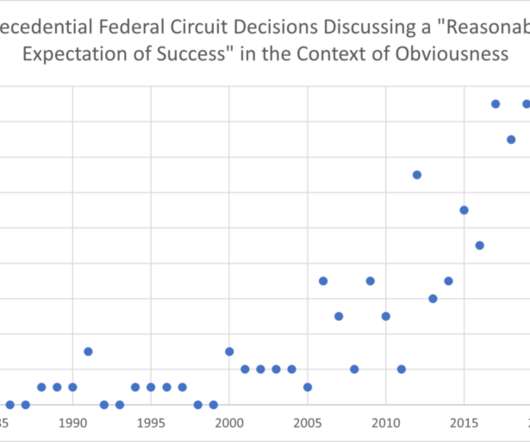
Overlapping Ranges in Claims and Prior Art Result in Invalidation of Patent on Transdermal Patch for Parkinson’s Disease
Patently-O
APRIL 15, 2023
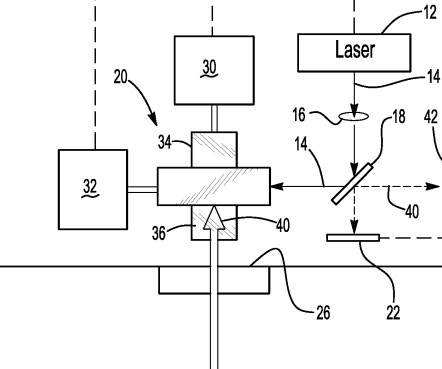
In 2007, UCB invented and marketed “original Neupro,” a transdermal patch for the treatment of Parkinson’s disease containing a dispersion of amorphous rotigotine and polyvinylpyrrolidone (PVP), with the PVP functioning as a stabilizer. market in April 2008 (although it remained in limited use under a compassionate-use program).



































Let's personalize your content