USPTO Provides Guidance in Light of Amgen v. Sanofi
JD Supra Law
JANUARY 19, 2024
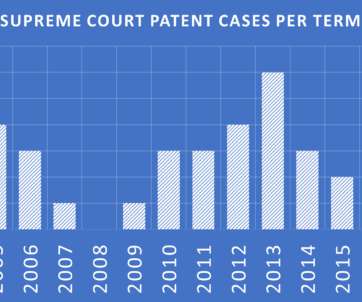
Supreme Court’s May 2023 decision in Amgen, Inc. Sanofi (Amgen) sent shock waves through the patent world, particularly in the chemical and biotech segments, due to its invalidation of Amgen patents based on a lack of enablement.


























Let's personalize your content