3 Count: Notorious Markets 2022
Plagiarism Today
MAY 2, 2022
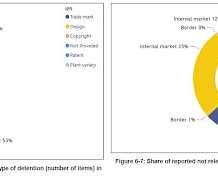
First off today, Chris Cooke at Complete Music Update reports that the United States Trade Representative has released its annual list of “notorious markets” that identifies countries that, according to it, are failing to take adequate action to prevent copyright and other kinds of intellectual property infringement.








































Let's personalize your content