News express: China releases a 15-year blueprint on the development of intellectual property rights (2021–2035)
The IPKat
SEPTEMBER 28, 2021
On 22 September 2021, China released a 15-year plan to develop intellectual property rights (IPR): ‘The Outline of Building a Powerful Intellectual Property Nation’ (2021–2035). The Outline’ (2021–2035) is highly noteworthy, comparable to the 2008 Outline of the National Intellectual Property Strategy. ‘The




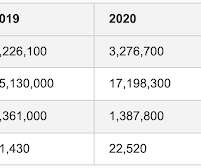











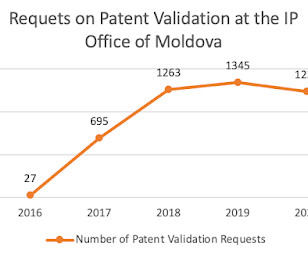


























Let's personalize your content