Public-Use Bar: What Startups Need to Know
IP Watchdog
OCTOBER 26, 2023
However, fewer startups are aware of the public-use bar and how activities pursued with the goal of growing their businesses may unwittingly invoke it.

IP Watchdog
OCTOBER 26, 2023
However, fewer startups are aware of the public-use bar and how activities pursued with the goal of growing their businesses may unwittingly invoke it.
IP Watchdog
FEBRUARY 15, 2023
Court of Appeals for the Federal Circuit (CAFC) on Wednesday issued a precedential opinion clarifying the requirements for the disclosure of technology that is ready for patenting at a public event to qualify as being “in public use” for purposes of the pre-America Invents Act (AIA) public use bar under 35 USC 102(b).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

JD Supra Law
MARCH 7, 2023
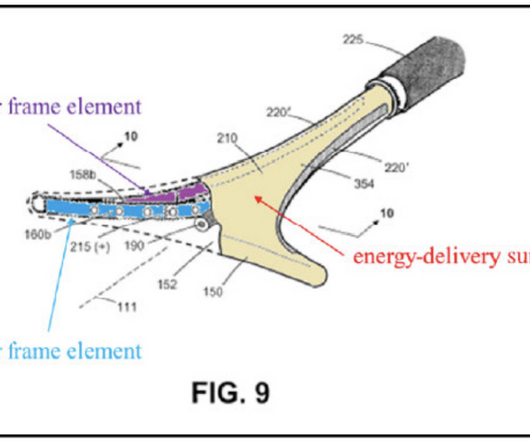
Minerva”) had engaged in an invalidating public use more than one year before its patent filing. . By: Irwin IP LLP On February 15, 2023, the Federal Circuit (“CAFC”) affirmed a summary judgment ruling that, by merely showcasing an embodying device at an industry event (the “Event”), Minerva Surgical, Inc.

IP and Legal Filings
MAY 10, 2024
While facilitating technology transfer, it is significant to look at how IP rights play a role. It’s the first important step towards protecting owner’s rights and its lawful public use. Intellectual Property Rights Protection IP licensing is an essential element of technology transfer. Why must an owner of IP license it?

JD Supra Law
FEBRUARY 22, 2023
9,186,208 on surgical devices for a procedure called endometrial ablation were anticipated under the public use bar of pre-AIA 35 U.S.C. § By: Harness IP Hologic, Inc., 2021-2246] (February 15, 2023), the Federal Circuit affirmed summary judgment that the asserted claims of U.S.

IP Law 360
MAY 15, 2024
and European patent decisions — concerning the effect of disclosures in clinical trials on the patentability of products — offers guidance on good practice for companies dealing with public use issues and prior art documents in these commercially important jurisdictions, say lawyers at Finnegan. A comparison of recent U.S.

LexBlog IP
FEBRUARY 21, 2023
9,186,208 on surgical devices for a procedure called endometrial ablation were anticipated under the public use bar of pre-AIA 35 U.S.C. § The Federal Circuit then pointed out that at the time of the public use, the technology was “ready for patenting.” § 102(b).
Let's personalize your content