How to Patent in China
Patent Trademark Blog
APRIL 5, 2023
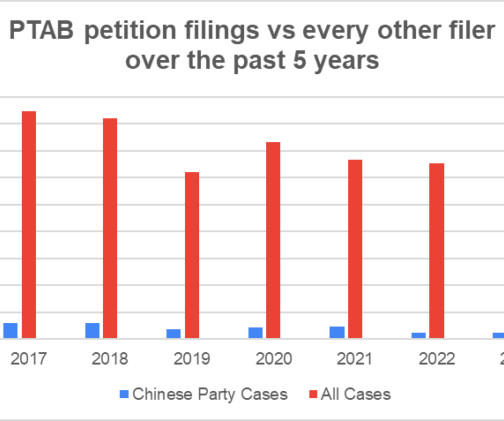
Does it make sense to get a patent in China? One of the most frequent questions I get about getting a patent in China is whether anyone should even bother. Why file a patent overseas when you cannot enforce it? Keep in mind that I am a US patent attorney. Need to file a patent in China?











































Let's personalize your content